November 15, 2021
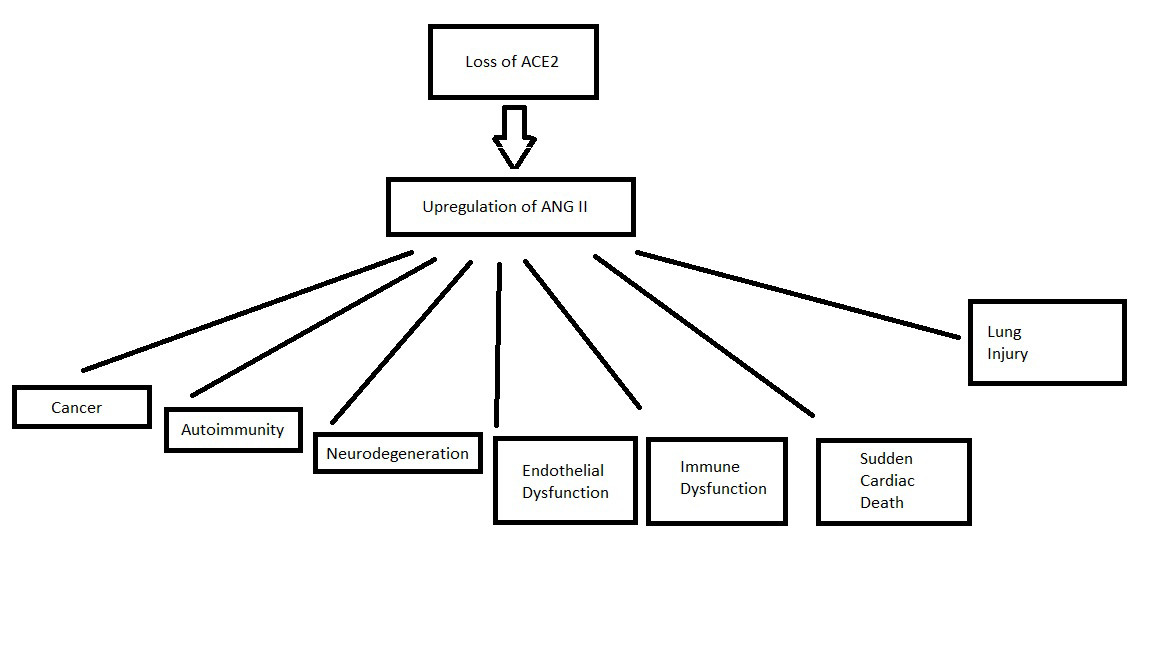
THE LOSS OF ACE2 RESULTS IN THE ULTRA-RAPID DEVELOPMENT AND PROGRESSION OF ACUTE AND CHRONIC AGE-RELATED DISEASES
If one were to be an enemy of humanity and wished to end the lives of as many people as possible and in the stealthiest way possible and could choose only one protein to remove from the body to accomplish this task – that protein would certainly be ACE2.
The loss of ACE2 causes a massive upregulation of Angiotensin II. This hormone is involved in all the pathologies of aging. Microvascular disease, cancer, neurodegeneration, heart disease, sudden death and immune dysfunction.
GROUND GLASS OPACITIES/LUNG INJURY
As an immediate concern, it is thought that the upregulation of ANG II is responsible for the ventilation-perfusion mismatch seen in COVID-19: We hypothesize that hypoperfusion of apparently healthy areas could be a consequence of vasoconstriction due to accumulation of angiotensin II, caused by decreased availability of ACE2, and that these changes in vascular resistance lead to a shunt or steal of vascular flow towards areas of non-aerated hyperperfused lung in moderate to severe COVID-19 cases.
Further studies are needed to investigate ventilation-perfusion abnormalities and whether these could be explained by the local increase in angiotensin II.
SUDDEN CARDIAC DEATH
Rats harboring the human renin and angiotensinogen genes (dTGR) feature angiotensin (ANG) II/hypertension-induced cardiac damage and die suddenly between wk 7 and 8. We observed by electrocardiogram (ECG) telemetry that ventricular tachycardia (VT) is a common terminal event in these animals.
IMMUNE DYSFUNCTION
The potential for angiotensin II to directly modulate inflammatory cell functions was suggested by the observation that human mononuclear leukocytes express specific binding sites for angiotensin II (27). Tsutsumi and associates subsequently demonstrated large numbers of binding sites for angiotensin II in rat spleen and found that these sites were primarily AT1 receptors (26). This is consistent with our receptor autoradiography findings of diffuse AT1-specific binding in mouse spleen, depicted in Figure Figure1a.1a. Our studies further show that these are predominantly AT1A receptors and that AT1A receptors are expressed in a variety of splenocyte populations including T cells, macrophages, and B cells.
Complementing its central role in BP homeostasis, the RAS has diverse and complex effects on innate and adaptive immunity. Through coordinated regulation of Ang II levels, the RAS proteolytic cascade affects hemodynamic injury in the heart, kidney, and vasculature leading to profound, global upregulation of inflammatory responses.
ENDOTHELIAL DYSFUNCTION
In the context of an inflammatory process, local activation of RAS and Ang II synthesis both increased vascular permeability by promoting the expression and secretion of VEGF (vascular endothelial growth factor) (Chua et al, 1998; Kitayama et al, 2006; Suzuki et al, 2003), and induced the expression of endothelial adhesive molecules including selectins (P- and L-selectin), vascular cell adhesion molecules-1 (VCAM-1), intercellular adhesion molecules-1 (ICAM-1) and their ligands, the integrins (Alvarez et al, 2004; Piqueras et al, 2000; Pueyo et al, 2000). Ang II also promotes endothelial dysfunction through COX-2 activation, which generates vasoactive prostaglandins and ROS (Welch, 2008; Wu et al, 2005).
AUTOIMMUNITY
The recent observation that Ang II modulates T cell responses, suggests a possible role of the peptide in autoimmune diseases. RAS is critically involved in the development of Th1/Th17-mediated multiple sclerosis (MS) as shown in experimental autoimmune encephalomyelitis (EAE), a well-established mouse model for human MS (Platten et al, 2009). Peripheral CD4+T cells from EAE mice show increased levels of Ang II which acting through the AT1 receptor promote the synthesis of Th1 and Th17 cytokines, specifically IFN-γ and IL-17.
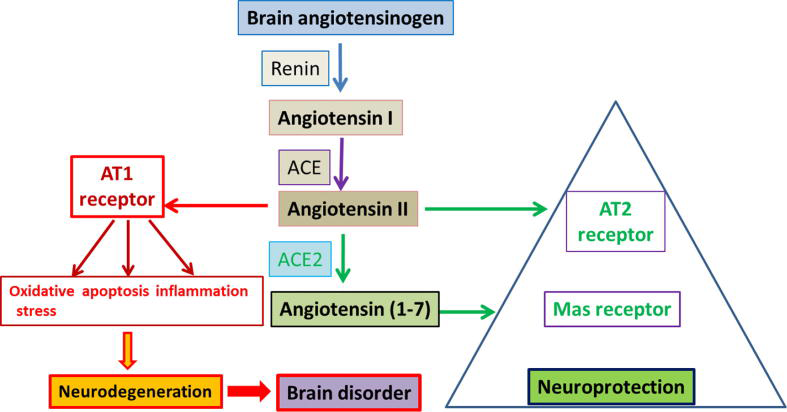
NEURODEGENERATION
As evidenced from literature, enzymes and peptides related to RAS are expressed and interact with other RAS components (Renin, Ang I, ACE, Ang II, AT1R and AT2R) in the brain. There is strong evidence for the activation of AngII/AT1R axis in initiating a cascade of events leading to increase in oxidative stress, apoptosis, and neuroinflammation causing neurodegeneration in the several brain disorders.
CANCER
The renin-angiotensin system (RAS) plays an important role not only in homeostasis but also in carcinogenesis. Recent epidemiological studies suggest that hypertensive patients with upregulated systemic RAS functions are at a significantly increased risk for the subsequent development of cancers with poor outcomes, and moreover that RAS inhibitors reduce tumor development, progression, and metastasis.
These hypotheses are coming to light, and just three days ago, a paper suggesting (as I have previously) that soluble ACE2 may be very therapeutic against COVID-19.
Referenced/Related Papers
Angiotensin II revisited: new roles in inflammation, immunology and aging
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3377325/
COVID-19: a hypothesis regarding the ventilation-perfusion mismatch
https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03125-9
Role of brain renin angiotensin system in neurodegeneration: An update
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7042626/
Role of renin-angiotensin system in gastric oncogenesis.
http://europepmc.org/article/PMC/3739298
Angiotensin II-induced sudden arrhythmic death and electrical remodeling
https://pubmed.ncbi.nlm.nih.gov/17416596/
Immunologic Effects of the Renin-Angiotensin System
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5407736/
Bioengineered angiotensin-converting-enzyme-2: a potential therapeutic option against SARS-CoV-2 infection