November 11, 2021
COVID-19 IS A DISEASE OF OVEREXPRESSED ANG II: ACE2 DEGRADING/DOWNREGULATION BY THE SPIKE PROTEIN RESULTS IN PRIMARY ARTERIAL HYPERTENSION, MICROVASCULAR DISEASE AND DNA DAMAGE.
THE SPIKE PROTEIN CAUSES HUMANS TO “SELF-INJECT” THEMSELVES WITH ANG II. JUST AS IS DONE IN LAB MICE EXPERIMENTS
Let us begin by looking at one of those very lab mice experiments using ANG II. In C57BL/6-mice equipped with osmotic mini pumps, delivering AngII in four different concentrations between 60 ng/kg per min and 1 μg/kg per min during 28 days, the oxidative and DNA damaging effects of AngII were studied, using immunohistochemistry and mass spectrometry.
The results?
AngII increased the SBP for up to 38 mmHg over control and adversely affected the kidney function of the mice. In the heart, at the highest dose administered, a significant increase of reactive oxygen species formation (1.4-fold over control) and double-strand breaks could be detected (13-fold over control). In the kidney, a dose-dependent increase of superoxide formation (up to 1.7-fold over control), double-strand breaks (up to 4.7-fold over control) and the mutagenic DNA base modification 7,8-dihydro-8-oxo-guanine (8-oxodG, up to 3.6-fold over control) was observed. Adverse effects already appeared at lower AngII doses, which did not raise the blood pressure. Administration of the radical scavenger tempol significantly decreased oxidative stress (by 20%) in the kidney and DNA double-strand breaks in the kidney by 60% and in the heart by 52%, without being able to lower the blood pressure.
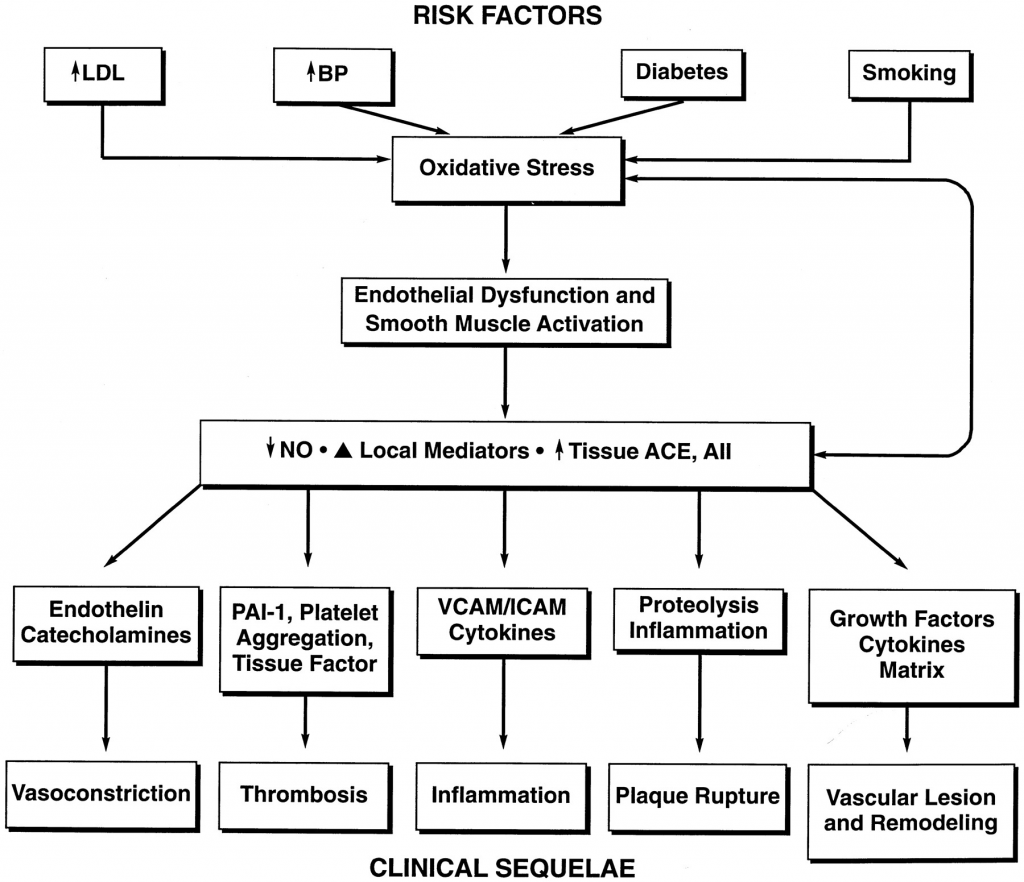
The artificially increased ANG II, caused by the depletion of its regulator, ACE2, results in the very pathological aspects of aging we have been observing in COVID-19. Specifically, that the renin–angiotensin system (RAS) is involved in regulation of blood pressure, vasoconstriction, sodium intake and potassium excretion is well established. Studies in the last few years have however documented new roles for this molecule as a pro-inflammatory molecule and more recently as a possible pro-fibrotic agent that contributes to progressive deterioration of organ function in disease. Binding of Ang II to its receptors (in particular AT1) mediates intracellular free radical generation that contributes to tissue damage by promoting mitochondrial dysfunction.
Furthermore, Angiotensin II is a potent mediator of oxidative stress and stimulates the release of cytokines and the expression of leukocyte adhesion molecules that mediate vessel wall inflammation. Inflammatory cells release enzymes (including ACE) that generate angiotensin II. Thus, a local positive-feedback mechanism could be established in the vessel wall for oxidative stress, inflammation, and endothelial dysfunction. Angiotensin II also acts as a direct growth factor for vascular smooth muscle cells and can stimulate the local production of metalloproteinases and plasminogen activator inhibitor. Taken together, angiotensin II can promote vasoconstriction, inflammation, thrombosis, and vascular remodeling.
ANG II overexpression is a complete textbook description of the “cytokine storm” and hyperactive immune response seen in COVID-19. In the context of an inflammatory process, local activation of RAS and Ang II synthesis both increased vascular permeability by promoting the expression and secretion of VEGF (vascular endothelial growth factor) (Chua et al, 1998; Kitayama et al, 2006; Suzuki et al, 2003), and induced the expression of endothelial adhesive molecules including selectins (P- and L-selectin), vascular cell adhesion molecules-1 (VCAM-1), intercellular adhesion molecules-1 (ICAM-1) and their ligands, the integrins (Alvarez et al, 2004; Piqueras et al, 2000; Pueyo et al, 2000). Ang II also promotes endothelial dysfunction through COX-2 activation, which generates vasoactive prostaglandins and ROS (Welch, 2008; Wu et al, 2005). Moreover, Ang II favours the recruitment of infiltrating inflammatory cells into tissues by stimulating the production of specific cytokine/chemokines. For example, Ang II induces the production of the potent monocyte chemoattractant MCP-1 in cultured monocytes (Dai et al, 2007). In the aorta of spontaneously hypertensive rats, which bear elevated levels of Ang II, massive macrophage infiltration is accompanied by increased expression of MCP-1 and one of its receptors, the C–C chemokine receptor CCR2. Modulation of MCP-1/CCR2 via AT1 receptor blockade reduces vessel inflammation in these rats (Dai et al, 2007).
This also explains the de novo onset of Diabetes being observed. Systemic RAS overactivation via gene overexpression or chronic Ang II infusion also induces insulin resistance, but not necessarily obesity.
Perhaps the most unsettling aspect of all of this is that the connection was observed in OCTOBER OF 2020.
We must immediately cease all Spike Protein exposures. The damage already done is incalculable.
Referenced/Related Papers
Overproduction of Angiotensinogen from adipose Tissue Induces adipose Infammation, Glucose Intolerance, and Insulin Resistance
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC4465436/
Angiotensin II revisited: new roles in inflammation, immunology and aging
https://www.embopress.org/doi/full/10.1002/emmm.201000080
Tissue Angiotensin and Pathobiology of Vascular Disease
https://www.ahajournals.org/doi/full/10.1161/01.HYP.37.4.1047
Angiotensin II-induced hypertension dose-dependently leads to oxidative stress and DNA damage in mouse kidneys and hearts
Small Resistance Artery Disease and ACE2 in Hypertension: A New Paradigm in the Context of COVID-19
https://www.frontiersin.org/articles/10.3389/fcvm.2020.588692/full